Shakuyakukanzoto mitigates adverse effects of chemotherapy—Investigator-initiated clinical trial and the first trial by the University of Toyama—

【Research Theme】
An investigator-initiated clinical trial about the effects of Shakuyakukanzoto on muscular and joint pain induced by paclitaxel
【Background】
Much pharmaceutical development is conducted by companies because they spend a large amount of money on research and development. On the other hand, doctors lead clinical studies and conduct the activities, from planning efficacy tests to analyzing final effects, so that medicines can be developed to solve patients’ most serious issues from the point of view of doctors.
Up until now, we have prescribed Kampo to patients with typical symptoms, considering only the effects of Kampo described in some classical books. Therefore, the Institute of Natural Medicine of the University of Toyama has embarked on a clinical study led by doctors in order to scientifically demonstrate that Shakuyakukanzoto extract, a form of Kampo, mitigates the side effects of muscular and joint pain induced by paclitaxel. If our research leads to the approval in Japan of the efficacy of Shakuyakukanzoto, then it will increase the importance of Kampo for all pharmaceuticals and will result in its increased use in Japan and abroad.
【Research Contents】
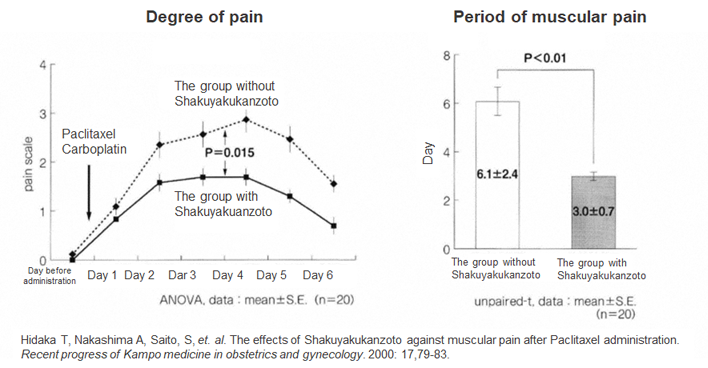
Paclitaxel is an anticancer agent used to treat ovarian cancer, non-small cell lung cancer, breast cancer, stomach cancer, uterine body cancer, esophageal cancer, and cervical cancer. It has been administrated to 400,000 patients annually in Japan. Though 30 to 40 percent of the patients with paclitaxel suffer from muscular and joint pain, general painkillers hardly reduce their pain, which is one of the major factors lowering their quality of life. The gynecology group from the department of Obstetrics and Gynecology at the University of Toyama has searched for a long time for pharmaceuticals that mitigate paclitaxel’s side effects, and it reports that Shakuyakukanzoto could mitigate them. Past research of patients with ovarian cancer using paclitaxel found Shakuyakukanzoto mitigated their muscular and joint pain and decreased the period of pain by half (Figure 1). However, no clinical studies of Kampo were conducted at that time 20 years ago, making it difficult for a new indication for Shakuyakukanzoto to be applied for or approval granted in Japan.
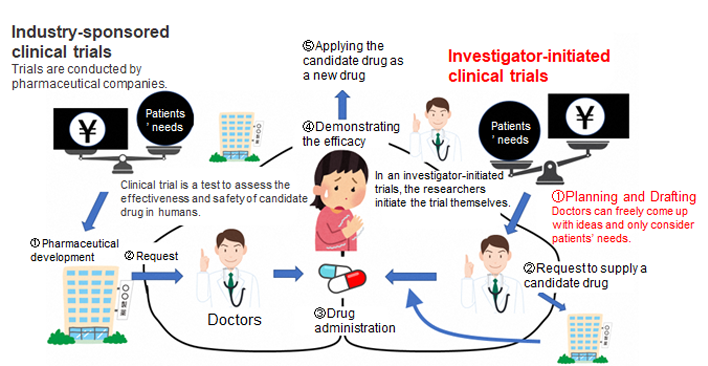
Some 20 years after the previous research, the University of Toyama, supported by Toyama Pharmaceutical Valley Development Consortium, restarted research in 2020 into whether Shakuyakukanzoto could mitigate the side effects of muscular and joint pain by paclitaxel. Its research is the first clinical study led by doctors at the University of Toyama. Although much pharmaceutical research is conducted by companies, they tend to prioritize highly profitable medicines due to their significant research and development costs (Figure 2, left). Considering this, we aim to supply medicines to patients in need who suffer from muscular and joint pain by paclitaxel regardless of market principles focused on profits. We can make this possible because our clinical study is led by doctors who will conduct all the steps concerning the efficacy of Shakuyakukanzoto, such as drafting and then notifying the authorities in Japan, conducting and controlling a trial plan, and then analyzing its results (Figure 2, right).
Supported by Toyama Pharmaceutical Valley Development Consortium, we created a system for an investigator-initiated clinical trial in cooperation with the Center for Clinical Research at Toyama University Hospital (Director Kazuyuki Tobe and Specially Appointed Professor Tsuyoshi Teramoto). We then drafted a trial plan and got approval for it from the Pharmaceutical and Medical Devices Agency. After one and a half years spent preparing our clinical study, we finally moved the plan forward. Considering that paclitaxel behaves as an anticancer agent against non-small lung cell cancer, breast cancer, stomach cancer and esophageal cancer, and gynecological cancer, several departments of Toyama University Hospital joined our study, including the First Department of Internal Medicine (Professor Kazuyuki Tobe and Clinical Associate Professor Minehiko Inomata), the Department of Surgery & Science (Professor Tsutomu Fujii and Assistant Professor Koshi Matsui), the Department of Clinical Oncology (Professor Ryuuji Hayashi and Assistant Professor Shinya Kajiura), the Department of Urology (Professor Hiroshi Kitamura), the Department of Japanese Oriental Medicine (Professor Yutaka Shimada and Professor Naotoshi Shibahara) and the Department of Hospital Pharmacy (Professor Atsushi Kato). This has made our investigator-initiated clinical trial possible since it started in December 2021 even though few studies using Kampo have been conducted in Japan. We expect to achieve success in this clinical study and be able to supply in Japan the drug of Shakuyakukanzoto as an anticancer agent to patients who suffer from the side effects of paclitaxel as soon as possible.
Inquiries
Please get in touch with us